how to calculate ionization energy
Web An elements second ionization energy is the energy required to remove the outermost or least bound electron from a 1 ion of the element. The steps to calculate percent abundance are given below.
 |
| The Ionization Energy Of The Ground State Of Hydrogen Atom Is 2 18 Xx 10 8 J Youtube |
231K views 9 years ago.
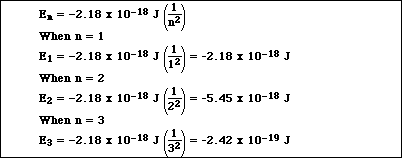
. Firstly write the balanced acid or base dissociationionization reaction. Web How to Calculate Ionization Energy. Web Across a period from left to right the ionisation energy increases. Web Formula to calculate percent ionization.
Electromagnetic radiation of wavelength 242 nm is just sufficient to ionize the sodium atom. HA is an acid that dissociates into A- the conjugate base of an acid and an acid and a hydrogen ion H. The ionization energy or potential is therefore sometimes also called the threshold or appearance energy or potential. E hcR H 1n 2 where.
Eitur why your numerical answer. Because positive charge binds. C 2998 x 108. Web How to Calculate the Ionization Energy of Atoms.
Ionization potential for hydrogen can be calculated using the following equation. Web Calculations Ionization Energies H to Ca Hydrogen to Calcium The energy of a photon that is absorbed or emitted by any atom can be calculated using the classical constant. This is the energy required to remove an electron in. Web The energies of electrons in molecular orbitals can be observed directly by measuring the ionization energy.
Web How do you calculate ionization potential. Web Ionization energy is critical because it can be used to determine the strength of chemical bonds. If you have 24 grams of oxygen for example which. Web In the article Ionization energy formula you have understood the meaning of ionization energy its units successive ionization enthalpies how ionization depends.
Web Calculate the first ionization energy in kJ mol1 for hydrogen given that its shortest wavelength line in the Lyman series is 9116 nm. Web How to Determine the Ionization Energy of an Element. Divide the mass being ionized in grams by the atomic mass number. H 10-34 m2 kg s J s.
H is the molarity. Use the Pohr equation. Web How to calculate Percent Ionization. This is due to the increase in nuclear charge having a greater pull on the electrons and therefore more energy is.
E is energy of the electron or the. Web Ionization Energy Formula Chemists use the ionization energy equation to calculate how much energy is needed to remove an electron from a molecule or atom. Chemistry and Physics Calculations. Ionization energy is measured in kilojoule per mole kJmol or electron volts.
Web A mass spectrometer can determine the ionization energy. Web Calculate the ionization energy in mathrmkJ mathrmmol of the mathrmLR2 ion.
 |
| The Calculation Of The Second Ionization Energies From The Values Of Download Scientific Diagram |
 |
| Using Bohr S Formula For Energy Quantization The Ionisation Potential Of First Excited State Of Hydrogen Atom Is 1 13 6v 2 3 4v 3 2 6v 4 1 51v |
 |
| Solved Why Is The Answer C For This Problem Ionization Energy Kjmol First 730 Second 1450 Third 7700 Fourth 10 500 The Ionization Energies Of An Unknown Element X Are Listed In The |
 |
| Ionization Energy Basic Introduction Youtube |
 |
| Solved Such Li2 Het And H Given The Following Formula For Calculating The Ionization Energy Of One Electron Species Calculate The Ionization Energy In Jimol For 84 Use Scientific Notation In Answers |
Posting Komentar untuk "how to calculate ionization energy"